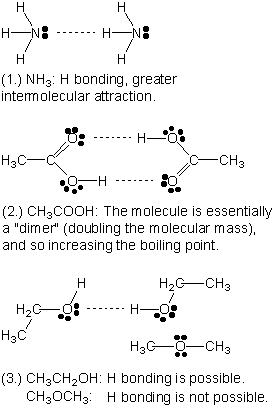
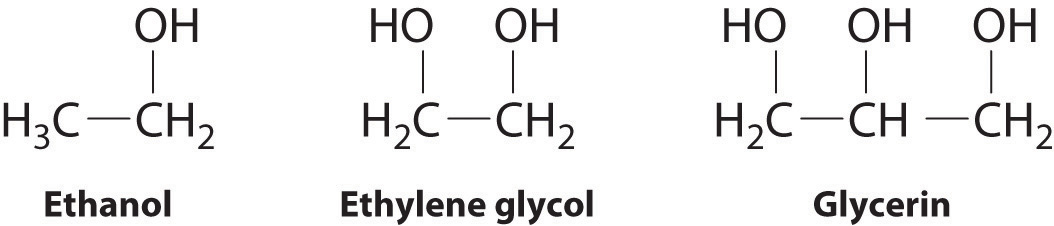Types Of Imf In Ethanol
wallpaperThe first force would be London Dispersion. All three of these forces are different due to of the types of bonds they form and their various bond strengths.
 Ethanol Intermolecular Forces In Ethanol
Ethanol Intermolecular Forces In Ethanol
Its chemical formula is C 2 H 6 O or can be written as C 2 H.
Types of imf in ethanol. London dispersion forces Keesom forces H-bonding. The SMS should not be used for sidestream blending. Heres a closer look at these three intermolecular forces with examples of each type.
In this experiment you will measure the evaporation rate of three different liquids. Intermolecular forces exist between molecules and influence the physical properties. List the following from lowest to highest melting point.
They are London dispersion dipole-dipole and the hydrogen bond. Intermolecular Forces in Ethanol. The second force would be Dipole Dipole see below.
This type of IMF which is weaker than H bonds is called dipole-dipole interactions. You might also be able to imagine an ion - dipole interaction. Solvents such as water or ethanol which are polar and capable of hydrogen bonding.
Ethanol also known as ethyl alcohol and abbreviated as EtOH is a colorless volatile and flammable liquid that is soluble in water. Ethanol is a clear liquid with a specific gravity of 0796 gasoline is 072-078 and a viscosity of 119cP compared with gasoline. There are three intermolecular forces which are collectively called van der Waals forces plus hydrogen bonding.
We can think of H 2 O in its three forms ice water and steam. Acetone molecules attract each other since they are both have permanent dipoles. This type of blending is termed sidestream or wildstream blending.
The Danload 6000 is capable of sidestream blending in both. Get 11 help now from expert Chemistry tutors. The type of intermolecular forces IMFs exhibited by compounds can be used to predict whether two different compounds can be mixed to form a homogeneous solution soluble or miscible.
London dispersion force dipole-dipole interaction and ion-dipole interaction. In general the molecules of alcoholic compounds such as isopropyl alcohol methanol ethanol and propyl alcohol combine through hydrogen bonding. Acetone ethanol and hexane.
There are three major types of intermolecular forces. Each group will also determine the evaporation rate of 2 unknowns. You will use your knowledge of Lewis structures molecular geometry and polarity to determine the types of IMFs in the liquids.
If you were to put 2 Ethanol molecules next to each other they would have 3 types of intermolecular forces bonding them together. Ethylene glycol the simplest member of the glycol family of organic compounds is commonly used as an antifreeze in automobiles. How to draw line angel structures write the IMF and circle the strongest type of IMF for each of these.
Dipole - dipole interaction top flash diagram ion - dipole H bond EthanolWater Solubility. Ether phenyl secondary alcohol tertiary alcohol tertiary amine primary amine secondary amine aldehyde ester aromatic amine. Previous question Next question Get more help from Chegg.
Aluminum oxide lithium chloride calcium chloride. A solution of water and ethanol contains the dipole-dipole forces and hydrogen bonds as the intermolecular forces between molecules. This happens between all molecules no matter what see below.
LiCl CaCl 2 Al 2 O 3 1-1 2-1 3-2 20. In organic reactions that occur in the cytosolic region of a cell the. There are three intermolecular forces of ethanol.
Methane trifluoromethane ethanol water lithiu chloride molecules with weakest IMF on left ionic compounds 19. A special type of dipole intermolecular forces called hydrogen bonding occurs when a hydrogen atom of one molecule chemically combines with either an oxygen or nitrogen atom of another molecule. Identification of intermolecular forces operating within liquid samples of water ethanol and acetone and the correlation of a physical property rate of evaporation with the type and strength of the IMF in the liquid.
Ethanol propanol pentane butyl hexane methanol water. Intramolecular forces bonding forces exist within molecules and influence the chemical properties. Trifluoromethane ethanol lithium chloride.
In all three cases the bond angles are the same the dipole moment is the same the molecular shape is the same and the.
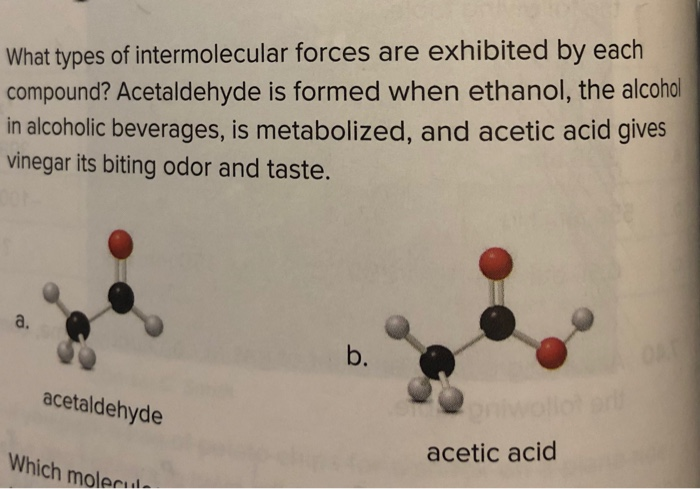 Solved What Types Of Intermolecular Forces Are Exhibited Chegg Com
Solved What Types Of Intermolecular Forces Are Exhibited Chegg Com
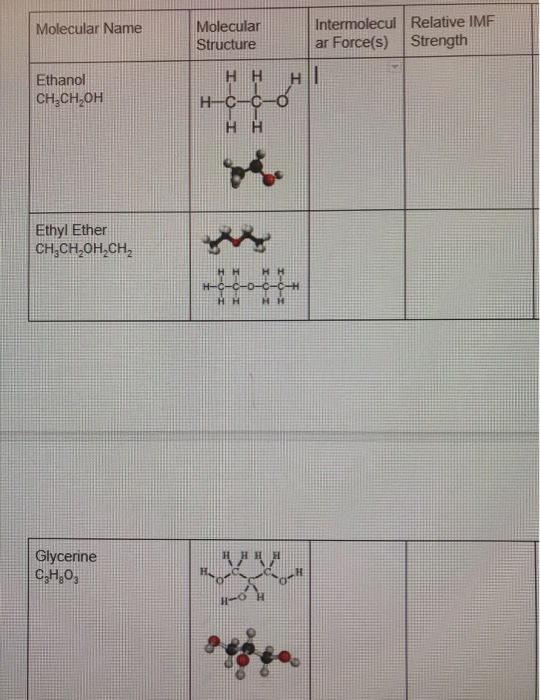 Solved Molecular Name Molecular Structure Intermolecul Re Chegg Com
Solved Molecular Name Molecular Structure Intermolecul Re Chegg Com
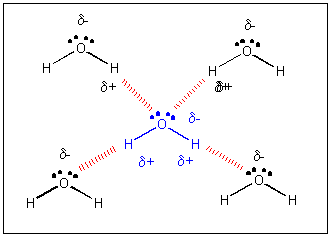 Intermolecular Forces And Solutions
Intermolecular Forces And Solutions
 Solved Post Lab Questions 1 What Type Of Intermolecular Chegg Com
Solved Post Lab Questions 1 What Type Of Intermolecular Chegg Com
 Intermolecular Forces Demonstration Relative Evaporation Rates Of Volatile Liquids Chemdemos
Intermolecular Forces Demonstration Relative Evaporation Rates Of Volatile Liquids Chemdemos
Https Pubs Acs Org Doi Pdf 10 1021 Acs Jchemed 5b00169
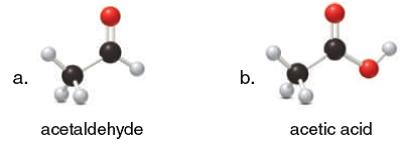 Solved Intermolecular Forces What Types Of Intermolecula Chegg Com
Solved Intermolecular Forces What Types Of Intermolecula Chegg Com
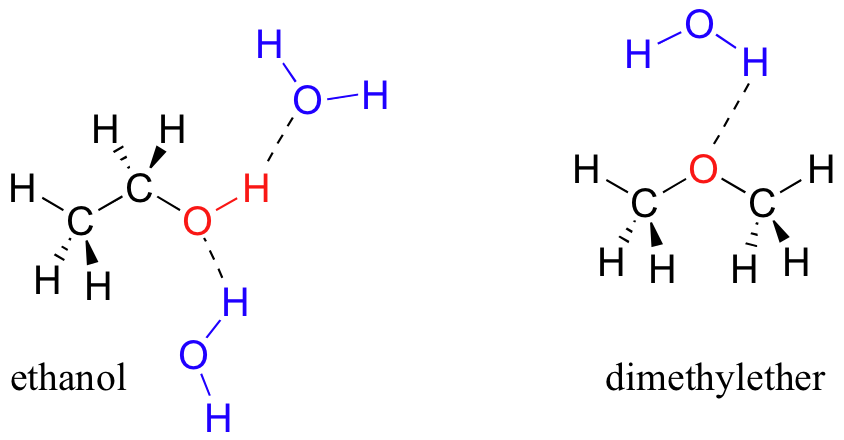 2 12 Intermolecular Forces Solubilities Chemistry Libretexts
2 12 Intermolecular Forces Solubilities Chemistry Libretexts
Physical Properties Of Alcohols Easy Exam Revision Notes For Gsce Chemistry
 Solved The Structures Of Ethanol And 1 Butanol Are Shown Chegg Com
Solved The Structures Of Ethanol And 1 Butanol Are Shown Chegg Com
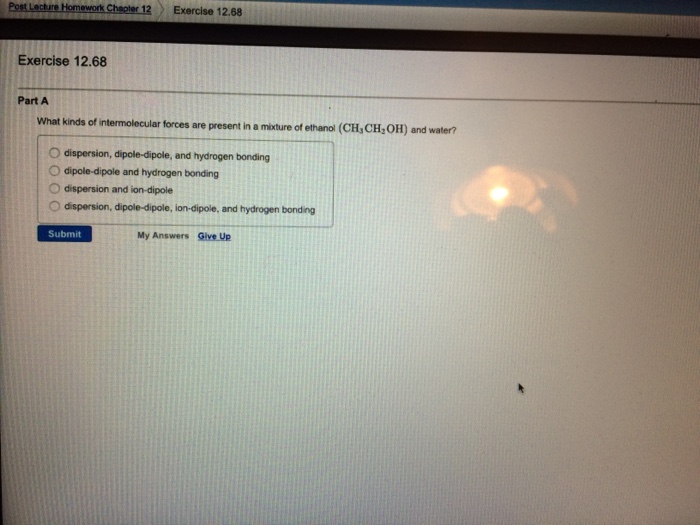
 Solved 1 What Type Of Intermolecular Force Is Predominan Chegg Com
Solved 1 What Type Of Intermolecular Force Is Predominan Chegg Com
 Solved 6 What Intermolecular Force S Of Attraction Is A Chegg Com
Solved 6 What Intermolecular Force S Of Attraction Is A Chegg Com
 Discovery Intermolecular Forces And Physical Properties
Discovery Intermolecular Forces And Physical Properties
 Solved Why The Ans Is D And What Is The Differnce Of Eac Chegg Com
Solved Why The Ans Is D And What Is The Differnce Of Eac Chegg Com
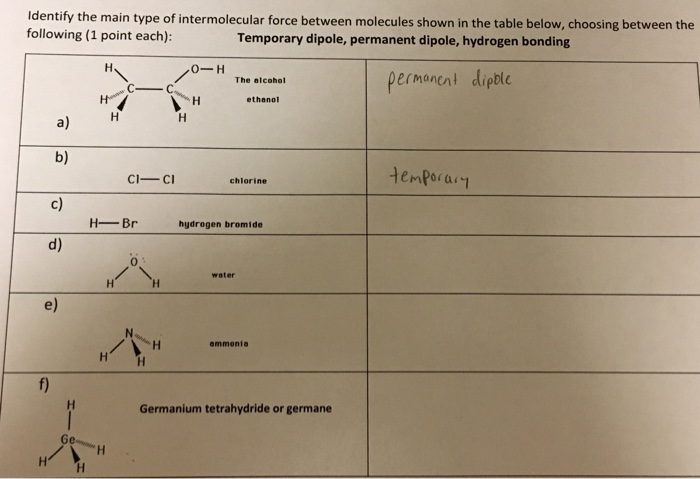
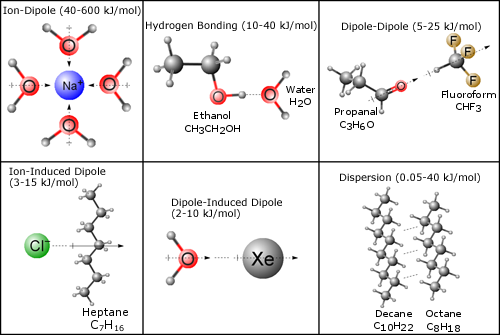 Solutions And Attractive Forces
Solutions And Attractive Forces
 Intermolecular Forces And Molecular Models Activity Chemdemos
Intermolecular Forces And Molecular Models Activity Chemdemos